
It can be used to derive the composition of a specific material. Constitutes of coloured lines which can be seen in the spectrum. When light passes through gas in the atmosphere some of the light at particular wavelengths is scattered resulting in darker bands. I think this is when white light is used that you get an Absorption Spectra. One of the major differences between the absorption spectrum and the emission spectrum is that the absorption spectrum has dark lines and the emission spectrum has different coloured lines. Emission lines refer to the fact that glowing hot gas emits lines of light, whereas absorption lines refer to the tendency of cool atmospheric gas to absorb the same lines of light. All the colors of the Absorption Spectra do make it kind of confusing.
ATOMIC EMISSION SPECTRUM VS CONTINUOUS SPECTRUM SERIES
And these are being absorbed (with emphasis on blue). If the gas is made incandescent by heat or an electric discharge, the resulting spectrum is a bright-line, or emission, spectrum, consisting of a series of bright lines against a dark background. Actually, if you just burned hydrogen and looked at its spectra, you would get the Emission Spectra and not the Absorption Spectra, and this Emission Spectra would only show the bunch of blue lines, one purple line, and one red line. A spectrometer is an instrument that is used to study the absorption and emission of light.

All the other colors shown are just part of the natural light being shown down on the element. This is the color that will be the opposite of the flame color on the color wheel.
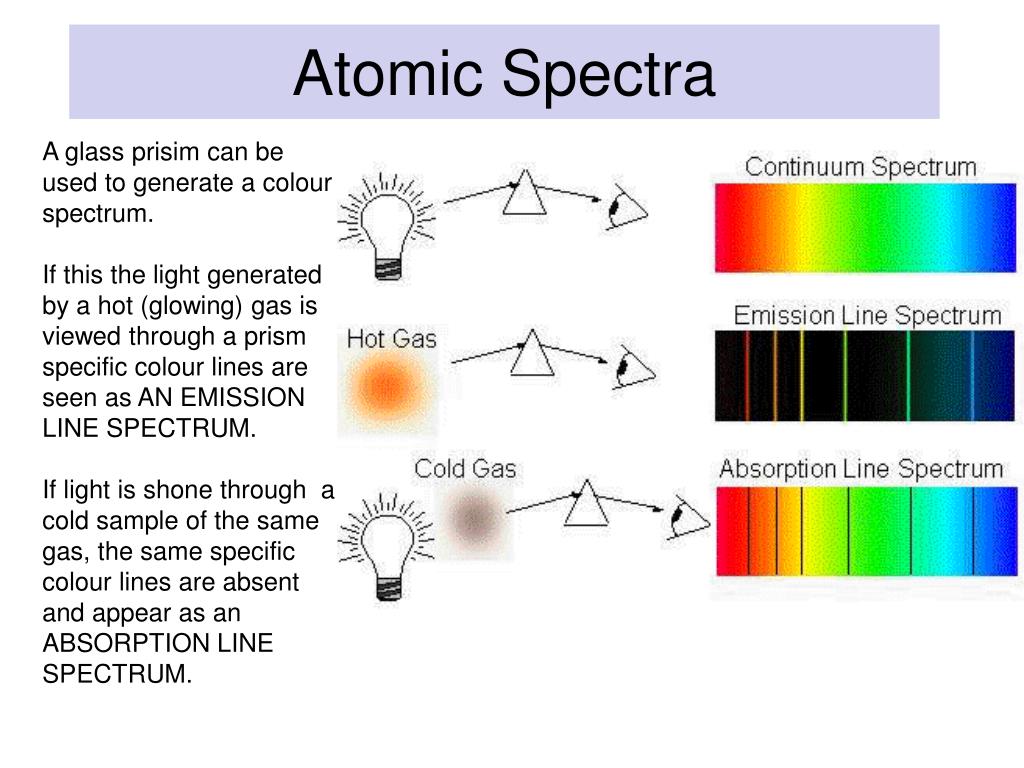
Remember, always look at the color area on the rainbow that is blacked out the most. Step 3: Now, lets compare this with a continuous. Three years later, Rydberg generalized this so that it was possible to determine the wavelengths of any of the lines in the hydrogen emission spectrum. So if blue is being absorbed, the opposite color would be transmitted and this color is orange. The color of the light emitted corresponds to the specific energy difference between the two levels. However, there are MORE dark lines in the blue region. In the case of the hydrogen atom the spectrum has both a continuous and a discrete part, the continuous part representing the ionization. If you look at the lines for hydrogen blue, purple, and red are being absorbed. Therefore, all the other colors would be absorbed. white light through a gas certain colors of light are absorbed by the gas, causing black bars to appear. The spectrum of visible light can be seen when white light is shined through a prism. (This would be orange.) The element hydrogen turns orange when being burned and this color is transmitted to us. The continuous spectrum definition is all of the values in the spectrum without any gaps, skips, or breaks. This means that if there is a big dark band where blue would be, then the opposite color to blue on the color wheel is being transmitted. A continuous spectrum is a combination of the absorption spectrum and emission spectrum, on the other hand, a line spectrum has either emission or an absorption.
You are supposed to look at the dark areas of the absorption spectra and those dark areas indicate that the color which would be there is being absorbed. The continuous spectrum has no gaps, breaks, or sharp lines in a given range of frequencies or wavelengths while the line spectrum contains clear gaps or lines between colored regions.

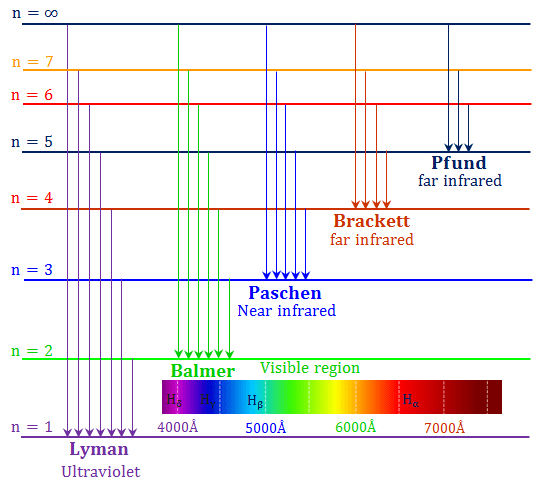
Identify what type of spectrum (continuous, emission, or absorption) would be produced in. Figure : When light from a hydrogen gas discharge tube is passed through a prism, the light is split into four visible. The figure below shows the atomic emission spectrum of hydrogen. Fellgett, an early advocate of the method. Below are three scenarios of light traveling to your spectrograph. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. excited state: A state where the potential energy of the atom is higher than the ground state. Infrared spectrum of ethanol.I think both the absorption and emission lines are showing which colors are being absorbed. One of the most important advantages of Fourier-transform spectroscopy was shown by P. continuous spectrum: All wavelengths of light are present.


 0 kommentar(er)
0 kommentar(er)
